 PhD Defense of Guillaume Lapouge on 10/09/2020
PhD Defense of Guillaume Lapouge on 10/09/2020


PhD Defense of Guillaume Lapouge from TIMC GMCAO team on October, the 9th at 2:30 pm:
« 3D ultrasound guided robotic needle steering. »
- Place : Auditorium bâtiment IMAG, 700 Avenue Centrale, 38400 Saint-Martin-d'Hères ⇒ obligatory reservation !
- Broadcast: https://youtu.be/hCyI5DXr6A4
 Thesis supervision :
Thesis supervision :
- Jocelyne TROCCAZ, Directrice de Recherche CNRS, laboratoire TIMC, Université Grenoble Alpes, Director
- Philippe POIGNET, Professeur des Universités, laboratoire LIRMM, Université de Montpellier, Director
 Jury :
Jury :
-
M. Emmanuel PROMAYON, Professeur, Université Grenoble Alpes, laboratoire TIMC, President
-
M. Brahim TAMADAZTE, Chargé de Recherche CNRS, Université Bourgogne Franche Comté, Femto-st, Reporter
-
M. Emmanuel VANDER POORTEN, Profresseur assistant, KU Leuven, Dept. of Mechanical Engineering, Reporter
-
M. Florent NAGEOTTE, Maître de Conférence, Université de Strasbourg, ICube, Examiner
-
M. Mahdi TAVAKOLI, Professeur, Univ. of Alberta, Dept. of Electrical and Computer Engineering, Examiner
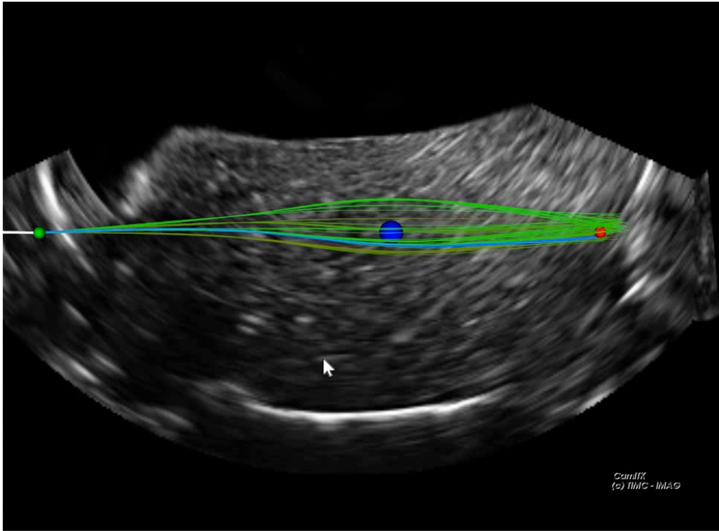
 Abstract :
Abstract :Robotic needle steering refers to the robotic guidance of a flexible needle as it is inserted into a tissue. Increasing the gesture precision, reducing tissue trauma and avoiding sensitive anatomical regions are among the expected advantages of this approach. However, despite sustained research efforts, it is not yet a clinical reality.
This thesis is a step towards such an objective, by contributing, at multiple levels, to a robust steering under 3D ultrasound imaging feedback. First, a needle localization algorithm is detailed. It enables the evaluation of the mechanical properties of the tissues that the needle navigates through. Besides, it adapts to the changing needle visibility in the 3D ultrasound volumes. Secondly, an efficient algorithm for the tracking of the targeted anatomical zone is elaborated. Thirdly, an adaptative path planner that considers uncertainties, noise and tissue heterogeneity is developped. It uses a priori knowledge of the tissue properties to predict the future needle deflection.
An experimental validation supports the performance of these approaches. The steering is characterized by an average targeting error of 1 mm, 1.5 ± 0.9 mm, and 1.7 ± 0.8 mm, in homogeneous phantoms, heterogeneous phantoms and biological tissue respectively. Finally, trials on an anatomical subject have provided prospects for the improvement of the proposed methods, to serve the ultimate purpose of a clinical application.
To conclude, robotic needle steering is still a work in progress. However, the work presented in this thesis stands out due to its its robustness, its compatibility with 3D ultrasound imaging and its patient-specific approach.
 Key words:
Key words:
Robot-assisted surgery, Needle steering, 3D ultrasound imaging, Image processing, Path planning, State observer
